Abstract
BACKGROUND: Individuals with immune mediated thrombotic thrombocytopenic purpura (iTTP) frequently exhibit cognitive impairment and are at higher risk of stroke than matched controls. However, the etiology of cognitive impairment in these individuals has not been established. We conducted this prospective study to examine the patterns of cognitive impairment and to test the hypothesis that cognitive impairment in iTTP survivors is associated with silent cerebral infarction (SCI, ischemic lesions on brain MRI without corresponding overt neurodeficits), which are also a risk factor for future stroke.
METHODS: We prospectively enrolled adult (age ≥ 18 years) patients with iTTP and age- and sex-matched controls without prior iTTP or stroke. iTTP was diagnosed based on ADAMTS13 activity <10% during an acute episode. All study assessments were performed after achieving hematological remission (platelet count >150x10 9/L for at least 30 days after stopping plasma exchange and/or caplacizumab). Participants undergo annual study visits including the following study measures: 1) NIH stroke scale to rule out acute stroke, 2) NIH ToolBox Cognition battery, a comprehensive iPad based neurobehavioral tool that has been validated across multiple populations and for which normative control data are available. The NIH ToolBox Cognition Battery tests multiple cognitive domains (Figure 1) including executive function (dimensional change card sort test, flanker inhibitory control and attention test), processing speed (pattern comparison test), episodic memory (picture sequence memory test), working memory (list sort working memory test), language (oral reading recognition, picture vocabulary test). We used T scores adjusted for age, sex, race and educational attainment where the normative mean T score is 50 with standard deviation (SD) of 10. Mild and major cognitive impairment are defined as a T scores that are 1-2 SD below mean and > 2SD below mean for any domain, respectively; 2) Brain MRI: SCI is defined as a infarct-like lesion (punctate T2 and FLAIR hyperintensity) without corresponding neurodeficits. The Chi-squared test and Mann Whitney test were used to compare categorical and continuous variables across groups, respectively.
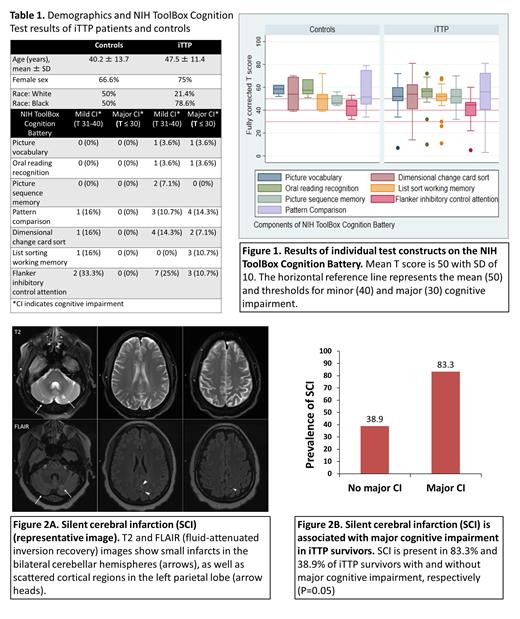
RESULTS: Between September 2020 and June 2021, we enrolled 34 participants including 28 iTTP patients and 6 controls. Demographics and clinical characteristics of patients and controls are summarized in Table 1. NIH Stroke Scale score was 0 in all controls and all but one iTTP patient (score = 1). Among iTTP patients, 28.6% (8/28) had prior stroke (including during acute episodes).
(i) iTTP survivors demonstrate cognitive deficits affecting executive function and processing speed: Among iTTP patients, 46.4% had cognitive impairment in at least one domain including 25% (7/28) with major cognitive impairment and 21.4% (6/28) with mild cognitive impairment (> 1 SD below mean T score). Among controls, 0% had major cognitive impairment and 16.6% (1/6) had mild cognitive impairment. Figure 1 shows detailed cognitive testing results for iTTP patients and controls. iTTP patients most commonly had deficits (T score < 40) in executive function (flanker inhibition test, dimensional change card sort test) and processing speed (pattern comparison test) (Table 1).
(ii) Silent cerebral infarction is associated with major cognitive impairment: MRI was completed in 24 iTTP patients and all controls. SCI was present in 50% (12/24) of iTTP patients (representative image, figure 2A) and 16.6% (1/6) controls. Another 29.1% (7/24) had evidence of prior stroke. Rate of SCI was 35.7% in patients without cognitive deficits, 50% in those with minor cognitive deficits and 83.3% in those with major cognitive deficits. SCI was present in 83.3% (5/6) with major cognitive impairment versus 38.9% (7/18) without major cognitive impairment (P = 0.05) (Figure 2B).
CONCLUSIONS: Cognitive deficits in iTTP survivors primarily affect executive function and processing speed and are associated with SCI suggesting ischemic etiology. Whether SCI occur during acute episodes or during remission (with remission ADAMTS13 as a risk factor) is being studied prospectively with serial MRI and cognitive assessment. iTTP patients may benefit from neurocognitive testing and referral to neuropsychological therapy, where appropriate, and aggressive measures to address risk factors for cerebrovascular disease.
Naik: Rigel: Research Funding. Lanzkron: GBT: Research Funding; Pfizer: Current holder of individual stocks in a privately-held company; Teva: Current holder of individual stocks in a privately-held company; Novartis: Research Funding; Novo Nordisk: Consultancy; Imara: Research Funding; CSL Behring: Research Funding; Shire: Research Funding; Bluebird Bio: Consultancy. Chaturvedi: Sanofi Genzyme: Other: Advisory board member; Argenx: Other: Advisory board member; UCB: Other: Advisory board participation; Dova: Other: Advisory board member; Alexion: Other: Advisory board member.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal